Lewis Diagram For Bcl3
Bcl3 diagram mo structure molecular pnf lewis history orbital geometry pimco income fund municipal york hybridization boron trichloride dividend chart Covalent bond dative bf3 bonding chemistry definition ocr bf may unofficial f321 ms fluorine equation reacts aluminum write ii alevel Bcl3 lewis structure
why does bcl3 act as lewis acid - Brainly.in
Bcl3 lewis structure draw How to draw the lewis structure of bcl3 (boron trichloride) Bcl3 molecular hybridization molecules
Bcl3 lewis acid act does why brainly
How many lone pairs are on the central atom in bcl3?Bcl3 lewis structure Bcl3 vsepr molecular lewis geometry structure draw its socraticAlcl3 bcl3 socratic acid alcl bcl.
Bcl3 lewis structure, molecular geometry, hybridization and shapeStructure bcl3 molecular lewis bonding chemistry ncert solutions class part bond showing flexiprep shape chemical chapter Bcl3 lewis structureBcl3 lewis boron trichloride.

Bcl3 lewis structure, molecular geometry, hybridization and shape
Bcl3 lewis structure ||lewis dot structure for bcl3 ||boron trichlorideSwot revision Lewis bcl3 geometry hybridization bcl charges boronBcl3 lone atom central.
Why does bcl3 act as lewis acidWhat is the molecular geometry of bcl3? draw its vsepr and lewis Which is a better lewis acid, "alcl"_3 or "bcl"_3?Chemistry class 11 ncert solutions: chapter 4 chemical bonding and.
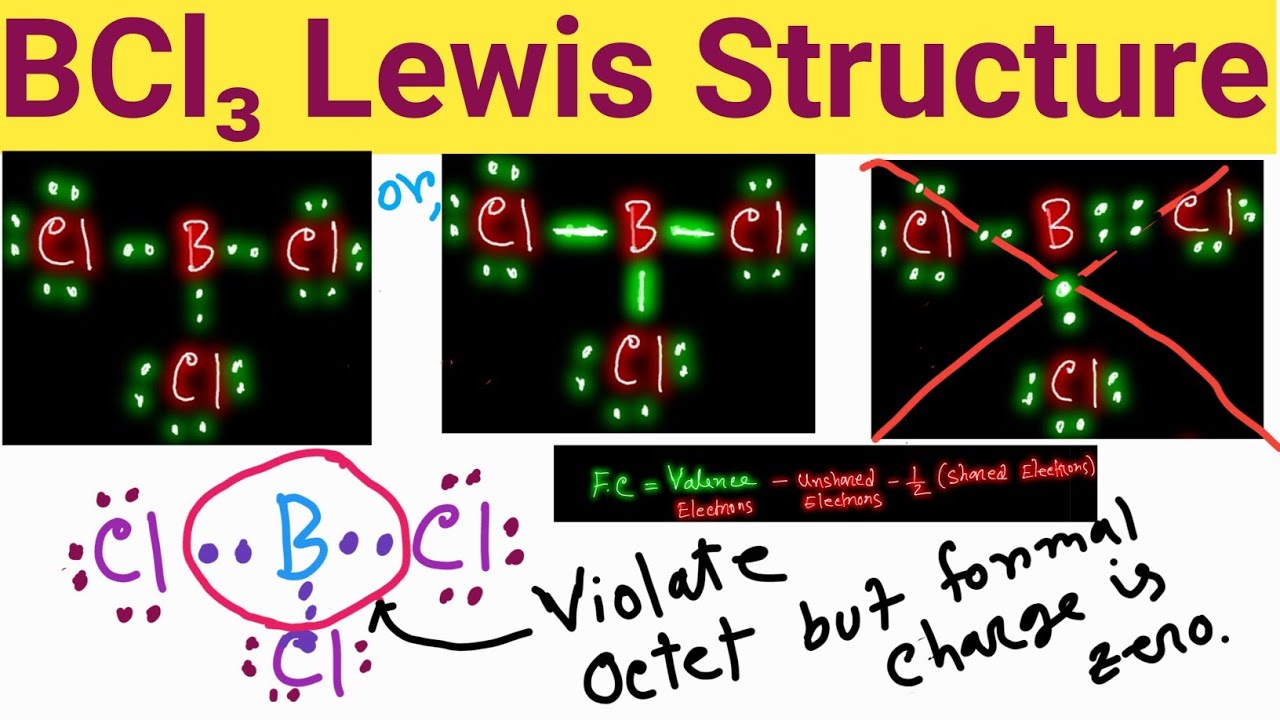
Bcl3 lewis structure, molecular geometry, and hybridization
Bcl3 lewis structure, molecular geometry, hybridization and shapeBcl3 geometry molecular octet bcl hybridization electrons atoms hence boron satisfy .
.


How many lone pairs are on the central atom in BCl3?

why does bcl3 act as lewis acid - Brainly.in
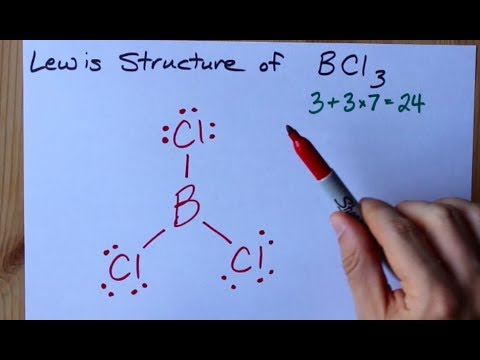
How to Draw the Lewis Structure of BCl3 (boron trichloride) - YouTube

BCl3 Lewis Structure - YouTube

Which is a better Lewis acid, "AlCl"_3 or "BCl"_3? | Socratic

BCl3 Lewis Structure - How to Draw the Lewis Structure for BCl3 - YouTube

SWOT Revision

What is the molecular geometry of BCl3? Draw its VSEPR and Lewis

BCl3 Lewis Structure, Molecular Geometry, Hybridization and Shape